octubre 2025
Noticias de Norteamérica
In the US, when hazards are identified in consumer products, they will be recalled and published in the Consumer Product Safety Commission (CPSC) Recent Recalls on the CPSC website, which is updated daily. The US recalls from 01 August 2025 to 31 August 2025 are summarized below:
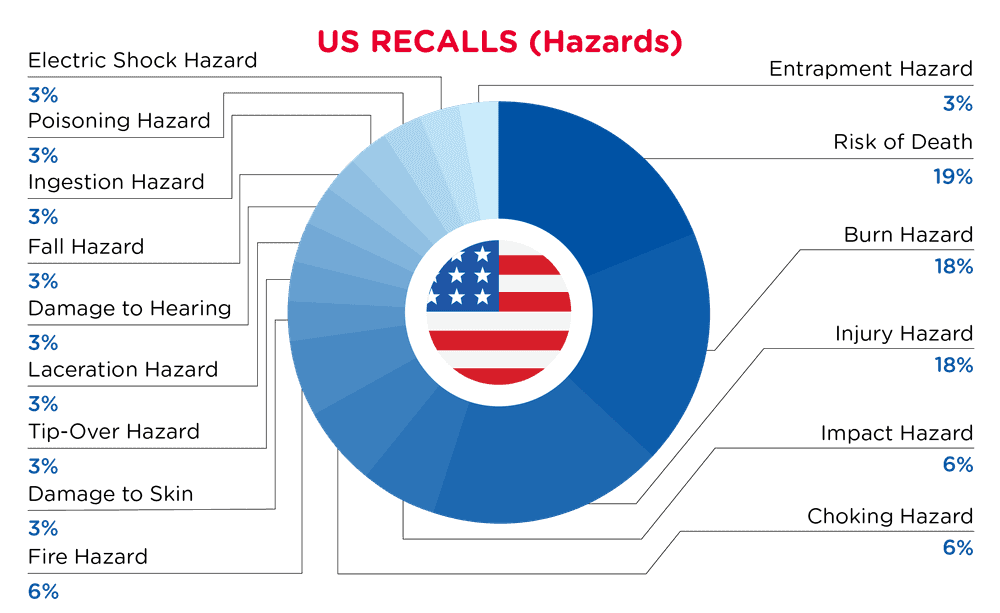
| Hazards | Frequency |
| Risk of Death | 6 |
| Burn Hazard | 6 |
| Injury Hazard | 6 |
| Impact Hazard | 2 |
| Choking Hazard | 2 |
| Fire Hazard | 2 |
| Damage to Skin | 1 |
| Tip-Over Hazard | 1 |
| Laceration Hazard | 1 |
| Damage to Hearing | 1 |
| Fall Hazard | 1 |
| Ingestion Hazard | 1 |
| Poisoning Hazard | 1 |
| Electric Shock Hazard | 1 |
| Entrapment Hazard | 1 |
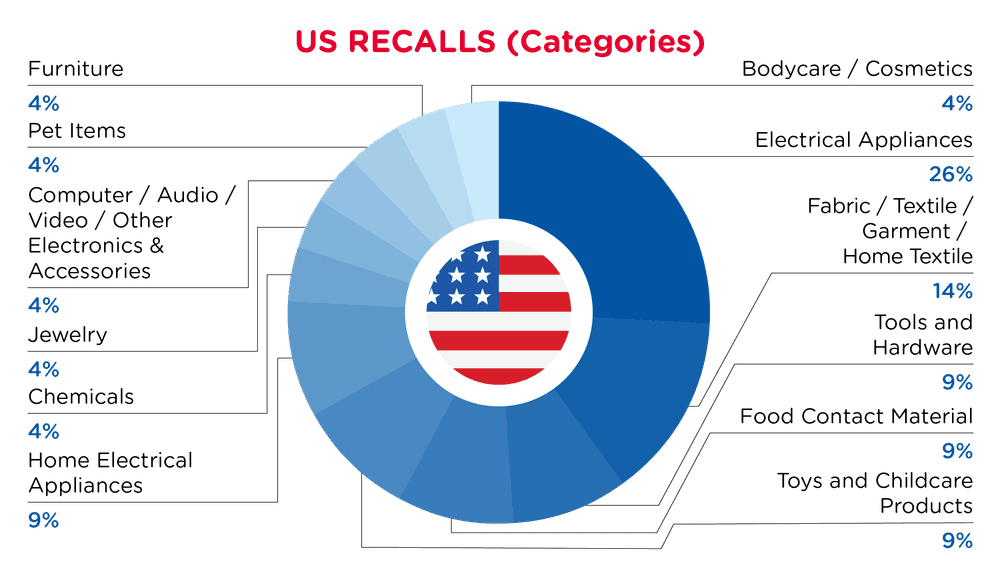
| Product Categories | Frequency |
| Electrical Appliances | 5 |
| Fabric / Textile / Garment / Home Textile | 3 |
| Tools and Hardware | 2 |
| Food Contact Material | 2 |
| Toys and Childcare Products | 2 |
| Home Electrical Appliances | 2 |
| Chemicals | 1 |
| Jewelry | 1 |
| Computer / Audio / Video / Other Electronics & Accessories | 1 |
| Pet Items | 1 |
| Furniture | 1 |
| Bodycare / Cosmetics | 1 |
For a complete list, click here
Washington State has introduced a new rule banning the intentional addition of formaldehyde and certain formaldehyde-releasing chemicals (known as formaldehyde releasers) in cosmetic products. This measure aims to protect public health by reducing exposure to these potentially carcinogenic substances. The regulation targets products sold, manufactured, or distributed within the state, including those used in professional services, online sales, and retail stores.
Formaldehyde is a colorless, strong-smelling chemical used historically in many industries as a preservative and disinfectant. In cosmetics, it was often added directly or indirectly (via formaldehyde-releasing preservatives) to prevent microbial growth and extend shelf life.
However, formaldehyde has raised serious safety concerns on its carcinogenicity, allergic reactions and inhalation risks in both occupational and consumer exposure scenarios.
In an effort to reduce exposure to these potentially carcinogenic substances, on 28 August 2025, the Washington Department of Ecology adopted a new rule, Chapter 173-399 WAC: Cosmetic Products Restriction, that expands the state's Toxic-Free Cosmetics Act (TFCA) to ban the use of formaldehyde and all formaldehyde-releasing chemicals in cosmetic and personal care products.
4. Scope
This rule applies to all cosmetic products intended for use on or in the human body for cleansing, beautifying, promoting attractiveness, or altering appearance. It covers:
Products sold in physical stores or online within Washington State.
Cosmetics used in professional services (e.g., salons).
Both finished products and those distributed for resale.
The ban specifically prohibits the intentional addition of formaldehyde or the listed releasers—trace impurities from other sources are not restricted.
5. Prohibited substances
The rule identifies 25 formaldehyde releasers that cannot be intentionally added. These are commonly used as preservatives or in formulations like nail polishes and hair products. The full list includes:
| Item | Substance Name | CAS number |
| 1 | DMDM Hydantoin | 6440-58-0 |
| 2 | Diazolidinyl Urea | 78491-02-8 |
| 3 | Imidazolidinyl Urea | 39236-46-9 |
| 4 | Quaternium-15 | 4080-31-3; 51229-78-8 |
| 5 | Tosylamide/Formaldehyde Resin (PTSAF) | 25035-71-6 |
| 6 | 2-Bromo-2-Nitropropane-1,3-Diol (Bronopol) | 52-51-7 |
| 7 | Sodium Hydroxymethylglycinate | 70161-44-3 |
| 8 | Polyoxymethylene Urea | 9011-05-6; 68611-64-3 |
| 9 | Polyoxymethylene Melamine | 9003-08-1 |
| 10 | 5-Bromo-5-Nitro-1,3-Dioxane (Bronidox) | 30007-47-7 |
| 11 | 7-Ethylbicyclo-oxazolidine (Bioban CS1246) | 7747-35-5 |
| 12 | Benzylhemiformal/td> | 14548-60-8 |
| 13 | Dimethylhydantoin Formaldehyde (DMHF) | 26811-08-5; 9065-13-8 |
| 14 | Dimethylol Glycol | 3586-55-8 |
| 15 | Dimethylol Urea | 140-95-4 |
| 16 | Dimethyl Oxazolidine | 51200-87-4 |
| 17 | MDM Hydantoin | 116-25-6; 27636-82-4; 16228-00-5 |
| 18 | Methenamine | 100-97-0 |
| 19 | Methylal | 109-87-5 |
| 20 | Paraformaldehyde | 30525-89-4 |
| 21 | Polyoxymethylene | 9002-81-7 |
| 22 | Tetramethylolglycoluril | 5395-50-6 |
| 23 | Timonacic (when used in heat-activated hair straighteners) | 444-27-9 |
| 24 | Tris-Hydroxymethylnitromethane | 126-11-4 |
| 25 | Urea, polymer with formaldehyde, isobutylated | 68002-18-6 |
6. Enforcement
| Requirement | Effective Date | |
| Formaldehyde releasers ban | No new manufacture, sale or distribution of cosmetics with intentionally added formaldehyde releasers | 1 January 2027 |
| Sell through of existing stock | Allow companies to comply and for retailers to manage existing inventory. After that, products must be off shelves | Allowed until 31 December 2027 |
The Washington State Department of Ecology will assess compliance via:
Review of product formulations.
Sampling and testing.
Other relevant data sources.
Labeling and ingredient disclosure should align with federal requirements, but state-specific verification may be needed.
In Canada, when hazards are identified in consumer products, they will be recalled and published in the Recalls and Safety Alerts Database on the Health Canada website, which is updated daily. The Canada recalls from 01 August 2025 to 31 August 2025 are summarized below:
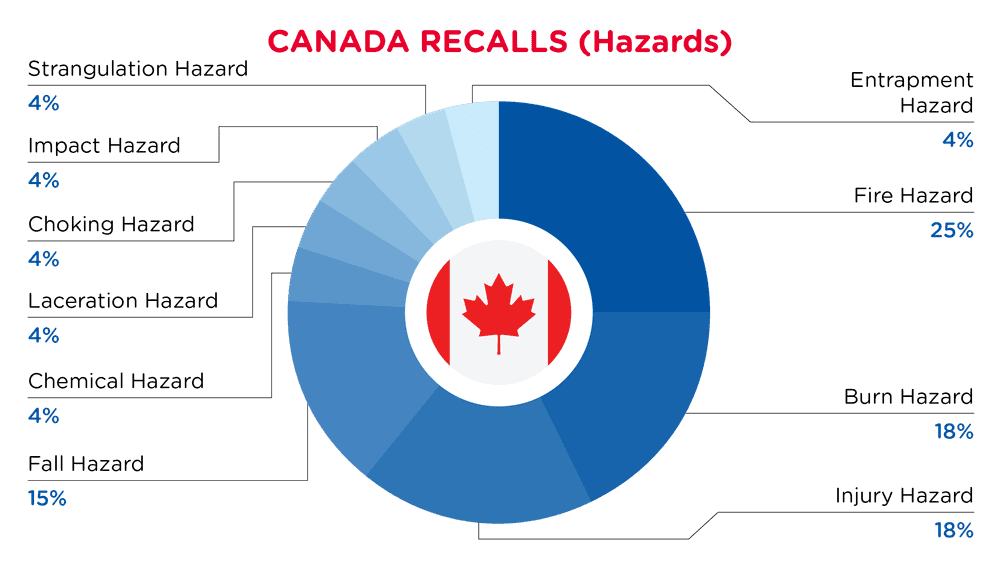
| Hazards | Frequency |
| Fire Hazard | 7 |
| Burn Hazard | 5 |
| Injury Hazard | 5 |
| Fall Hazard | 4 |
| Chemical Hazard | 1 |
| Laceration Hazard | 1 |
| Choking Hazard | 1 |
| Impact Hazard | 1 |
| Strangulation Hazard | 1 |
| Entrapment Hazard | 1 |
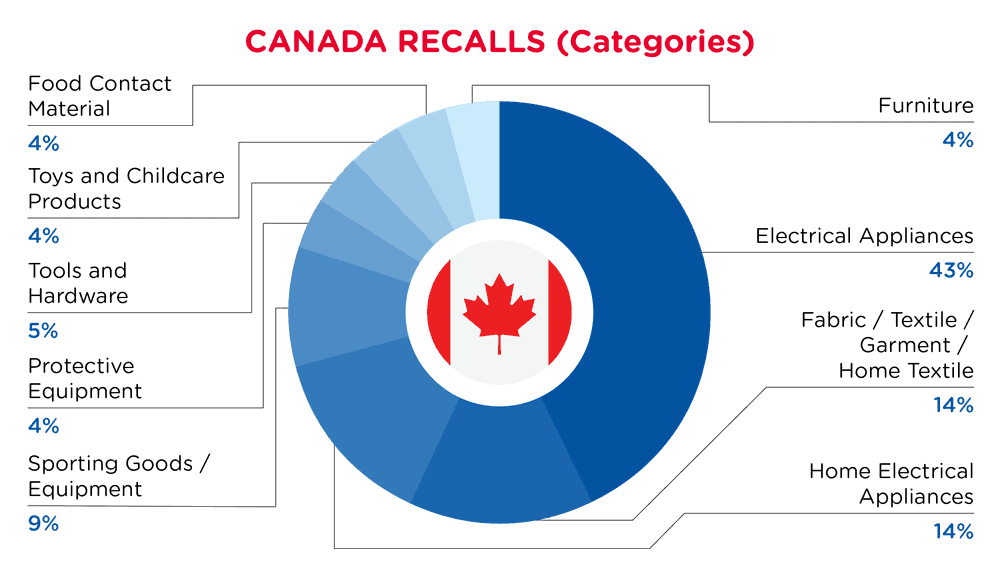
| Product Categories | Frequency |
| Electrical Appliances | 9 |
| Fabric / Textile / Garment / Home Textile | 3 |
| Home Electrical Appliances | 3 |
| Sporting Goods / Equipment | 2 |
| Protective Equipment | 1 |
| Tools and Hardware | 1 |
| Toys and Childcare Products | 1 |
| Food Contact Material | 1 |
| Furniture | 1 |
For a complete list, click here
Noticias de Europa
In Europe, when hazards are identified in non-food consumer products, the products will be recalled and published in the Safety Gate system, which is updated weekly. The European recalls from 01 August 2025 to 31 August 2025 are summarized below:
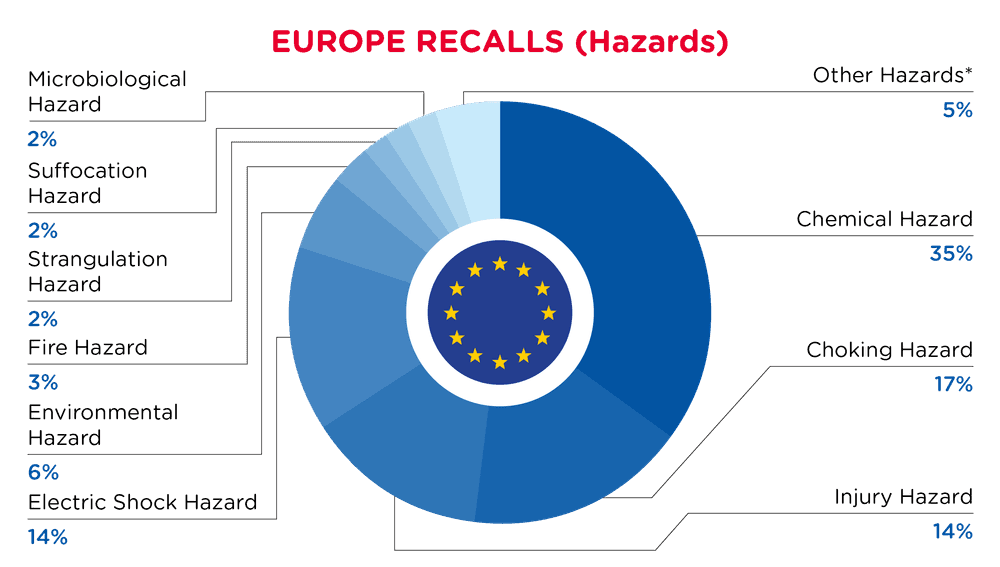
| Hazards | Frequency |
| Chemical Hazard | 108 |
| Choking Hazard | 54 |
| Injury Hazard | 43 |
| Electric Shock Hazard | 42 |
| Environmental Hazard | 18 |
| Fire Hazard | 11 |
| Strangulation Hazard | 8 |
| Suffocation Hazard | 5 |
| Microbiological Hazard | 5 |
| Other Hazards* | 15 |
*Other Hazards include Damage to Hearing, Burn Hazard, Damage to Sight, Entrapment Hazard and Health Risk Hazard with a frequency of less than 5.
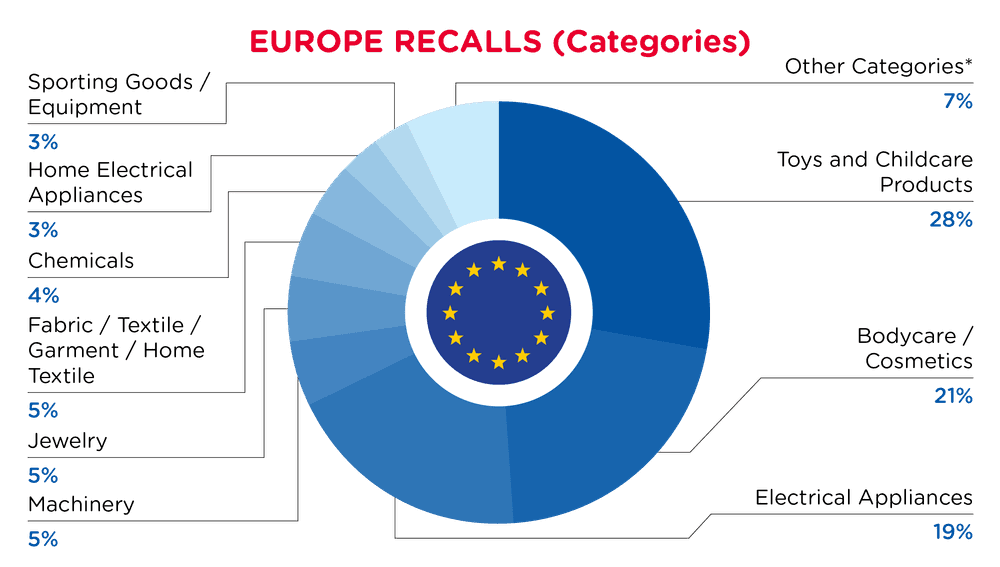
| Product Categories | Frequency |
| Toys and Childcare Products | 69 |
| Bodycare / Cosmetics | 52 |
| Electrical Appliances | 46 |
| Machinery | 13 |
| Jewelry | 12 |
| Fabric / Textile / Garment / Home Textile | 12 |
| Chemicals | 10 |
| Home Electrical Appliances | 8 |
| Sporting Goods / Equipment | 7 |
| Other Categories * | 17 |
*Other Categories include Stationery, Computer / Audio / Video / Other Electronics &, Accessories, Outdoor Living Items, Protective Equipment, Footwear, Pet Items, Furniture and Accessories with a frequency of less than 7.

| Notifying Country | Frequency |
| Hungary | 47 |
| Sweden | 32 |
| France | 28 |
| Czechia | 23 |
| Germany | 19 |
| Poland | 18 |
| United Kingdom in respect of Northern Ireland | 17 |
| Lithuania | 11 |
| Slovakia | 10 |
| Italy | 8 |
| Austria | 8 |
| Other Countries* | 25 |
*Other Countries include Ireland, Norway, Cyprus, Spain, Croatia, Finland, The Netherlands, Malta and Belgium with a frequency of less than 8.
For a complete list, click here
The European Commission has issued a draft amendment on 10 September 2025 to correct the terminology, scope and deadlines for the use of BPA in Regulation (EU) 2024/3190.
On 10 September 2025, the European (EU) Commission proposed a draft regulation to revise Commission Regulation (EU) 2024/3190 on the use of bisphenol A (BPA) and other bisphenols and bisphenol derivatives. This revision is to clarify certain inconsistences and errors, which are important to correct to ensure the proper execution of the Regulation.
The following table can be helpful to quickly review the revisions:
Table 1- Revisions of Commission Regulation (EU) 2024/3190.
| Article of (EU) 2024/3190 | Comparison of the revisions in paragraph | Note |
| Article 3 Prohibition of the use of BPA | 1. The use of BPA in the manufacture of food contact materials and articles……, is prohibited. 2. By way of derogation from paragraph 1, BPA may be used in the manufacture of food contact materials…… | In Article 3 of Regulation (EU) 2024/3190, the reference to ‘BPA and its salts’ is inconsistent with the definition of ‘bisphenol’ laid down in Article 2(2)(c) of that Regulation. Therefore, the words ‘and its salts’ should be deleted from Article 3. |
| Article 9 Verification of compliance with the requirements of this Regulation | 2. For the selection of methods used to verify that a food contact material or article does not contain residual BPA, another hazardous bisphenol or a hazardous bisphenol derivative…… (c) to verify that a food contact material or article does not contain residual BPA, another hazardous bisphenol or a hazardous bisphenol derivative, an extraction method shall be used. | Article 9(2) of Regulation (EU) 2024/3190 aims to ensure that an appropriate method of analysis is used to determine compliance with Article 4 of that Regulation. Since Article 4 prohibits the presence of ‘residual BPA’, Article 9(2)(c) of that Regulation should also refer to ‘residual BPA’. |
| Article 11 Transitional provisions concerning single-use final food contact articles | 1. Single-use final food contact articles manufactured using BPA and complying with the rules as applicable before the date of entry into force of this Regulation, which do not comply with the rules in this Regulation, may be first placed on the market until 20 July 2026. 2. By way of derogation from paragraph 1…… which do not comply with the rules in this Regulation, may be first placed on the market until 20 January 2028. | Article 11 of that Regulation aims to provide transitional provisions for the first placing on the market of single-use final food contact articles. Since that Article 11 however mistakenly refers only to food contact materials ‘placed on the market’, it should be corrected. |
| Article 12 Transitional provisions concerning repeat-use final food contact articles | 3. Repeat-use final food contact articles that were first placed on the market in accordance with paragraph 1 (may remain on the market until) 20 July 2027. Repeat use final food contact articles that were first placed on the market in accordance with paragraph 2 may remain on the market until 20 January 2029. | The end of the transitional period for food contact articles in paragraph 1 is different from the end of the transitional period for food contact articles in paragraph 2, the indicated date of 20 January 2029 only applies to articles first placed on the market in accordance with paragraph 2. |
| ANNEX III The declaration of compliance referred to in Article 8 shall contain the following information | (3) the identity of intermediate food contact materials final food contact articles; | The aim is to require the identity of food contact materials and articles from one business to another. Therefore, the wording should be corrected. |
This amendment shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
Manufacturers shall pay attention to the manufacturing and storage, to ensure they meet the updated requirements.
ECHA has announced a consultation phase for potential inclusion of three chemicals as SVHC. Should these listings be approved, the number of substances recognized as SVHC on the candidate list will be potentially updated to 254 entries.
On 1 September 2025, the European Chemical Agency (ECHA) launched a 45-days public consultation on three new potential Substances of Very High Concern (SVHC) as shown in the table below.
On 27 June 2025, the European Chemical Agency (ECHA) launched a 45-days public consultation on the substance decabromodiphenyl ethane (DBDPE), as a new potential SVHC.
If the three most recent substances are approved [together with DBDPE (CAS number: 84852-53-9)], the number of SVHCs on the Candidate List will be updated from 250 entries to 254 entries.
A decision will be made whether these substances will be included in the ECHA Candidate List of SVHC after the consultation period closes.
| Substance name | EC number | CAS number | Reason for inclusion | Possible usage |
| 4,4'-[2,2,2-trifluoro-1-(trifluoromethyl)ethylidene]diphenol and its salts | - | - | Toxic for reproduction (Article 57c) | Production of polymer and rubber (e.g., reactive process regulator, vulcanizing agents or cross-linkers) |
| 4,4'-methylenediphenol | 210-658-2 | 620-92-8 | Toxic for reproduction (Article 57c) | Substitute for other bisphenols in polymers, plastics or thermal paper Lacquers, varnishes, liners, coatings, adhesives, water pipes and dental sealants |
| n-hexane | 203-777-6 | 110-54-3 | Specific target organ toxicity after repeated exposure (Article 57(f) - human health) | Coating, polishes and wax blends Perfumes, fragrances Cosmetics, personal care products Cleaning agents, Blowing agents, Functional Fluids, Polymer processing |
The European Directorate for the Quality of Medicines & Healthcare recently issued the technical guide for food contact cork material and articles-2025 1st -edition.
On 1 September 2025, the European Quality of Medicines & Healthcare (EDQM) issued a new technical guide which supplements the Council of Europe Resolution CM/Res (2020)9 on the safety and quality of materials and articles for contact with food and supersedes the Council of Europe’s policy statement concerning cork stoppers and other cork materials and articles intended to come into contact with foodstuffs (Version 2 dated 05.09.2007).
The new EDQM Technical Guide makes important updates to the compliance requirements for food contact cork materials. The following table can be helpful to quickly review the core changes:
Table 1- Abstract of the EDQM Technical Guide
| Item | EDQM Technical Guide of Cork (core content and important changes) | Note |
| Scope and definitions | Applicable for food contact cork materials and articles. The main constituent of these materials and articles is cork, harvested from cork oak (ISO 633: 2019). | e.g. cork stoppers, trays, placemats and fruit bowls. |
| Specific requirements | No mandatory requirement with either the QMA* or Specific Migration Limit SML** for Pentachlorophenol (PCP) and Trichlorophenol (TriCP). * QMA is the maximum permitted residual quantity of a substance in the final material or article expressed as weight per surface area of the article in contact with food ** SML is the Specific Migration Limit | PCP and TriCP are general requirements in the previous Policy Statement. |
| Authorized substances | The list has been updated. | / |
| Compliance testing | Meet the restrictions applicable to the substances stated in the authorized list. Active substances may be used if these products have been authorized for their use. Not exceeding the maximum residue levels of pesticides in food as laid down in Regulation (EC) No 396/2005 (applicable to products of plant and animal origin or parts thereof to be used as fresh, processed and/or composite food or feed). Shall not contain any fungi, fungal toxins or bacteria. | / |
| Test conditions and methods of analysis | The food simulants and compliance testing conditions set out in Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food and the Joint Research Center (JRC) Guide in its publication Testing conditions for kitchenware articles in contact with foodstuffs shall be followed. | / |
| Declaration of compliance (DoC) | The EDQM Technical Guide emphasizes the importance of the DoC and lists the information that the DoC should contain. | / |
This guide can be applied immediately.
Manufacturers shall evaluate the cork formulations and production processes and pay attention to the Declaration of Compliance, to ensure they meet the updated authorized substance list and technical requirements.
The updated EU PFAS restriction proposal, published by ECHA on 20 August 2025, targets the manufacture, market placement, and use of over 10,000 PFAS substances under the REACH Regulation, focusing on 14 key sectors including consumer products like textiles, packaging, and cosmetics.
Moving away from a blanket ban, the proposal introduces flexible restriction options and emission controls to balance environmental protection with industry feasibility. These measures enhance manageability by allowing continued use in critical applications. The proposal requires consumer product industries to prepare for compliance through testing and alternative development during transitional periods.
Proposal opinions are expected in 2026.
On 20 August 2025, the European Chemicals Agency (ECHA) published an updated proposal to restrict per- and polyfluoroalkyl substances (PFAS)—commonly known as "forever chemicals"—under the EU's registration, evaluation, authorization and restriction of chemicals (REACH) Regulation. This revision follows an extensive review of over 5,600 stakeholder comments from the initial 2023 consultation. The updated report, known as the Background Document, will serve as the basis for the opinions of ECHA’s committees for Risk Assessment (RAC) and Socio-Economic Analysis (SEAC). Led by authorities from Denmark, Germany, the Netherlands, Norway, and Sweden, the proposal seeks to minimize PFAS emissions into the environment and protect human health by addressing risks from more than 10,000 PFAS substances across key sectors.
While the original proposal considered a broad "universal" ban, the update takes a more refined approach, introducing specific exemptions and phased implementation timelines to strike a balance between environmental goals and practical feasibility. This is particularly relevant for the consumer products industry, where PFAS are used in items like textiles, packaging, and cosmetics.
1. Scope
The restriction targets the manufacture, placing on the market, and use of PFAS in the EU, focusing on intentional additions to products and processes. It applies to a wide range of sectors, but due to evaluation timelines, the initial restriction will cover only the original 14 sectors identified in the 2023 dossier. Eight additional sectors, identified through consultation feedback, will not be included in the 2026 committee opinions and may require separate future assessments.
| Category | Sectors Covered (Original 14) | Sectors Excluded (8 New Sectors – Deferred) |
| Consumer Focused | - Textiles and apparel - Paper and packaging (e.g., food contact materials) - Cosmetics - Personal protective equipment (PPE) | - Printing applications - Technical textiles |
| Industrial and Technical | - Coatings and paints - Construction products - Lubricants - Electronics and semiconductors - Transport (e.g., vehicles) - Energy (e.g., heat transfer fluids, batteries) | - Sealing applications - Machinery applications - Broader industrial uses (e.g., solvents, catalysts) |
| Specialized | - Medical devices - Fluorinated gases and refrigerants | - Other medical applications (e.g., pharmaceuticals packaging) - Military applications - Explosives |
2. Restricted Options (ROs)
The original proposal set out two Restriction Options (RO): RO1, a complete PFAS ban with an 18-month transition, and RO2, a ban with sector-specific derogations of 5 to 12 years, also preceded by an 18-month transition. In response to stakeholder input, the authorities additionally examined a third option (RO3), which would allow certain uses of PFAS to continue under strict lifecycle-based emission controls.
The updated proposal evaluates these restriction options (ROs) emphasizing risk-based controls. The core approach is a general prohibition on PFAS, with tailored derogations to allow continued use where alternatives are unavailable, or risks can be adequately managed.
| Restricted Option | Description | Coverage/Restrictions |
| RO1: Full Ban | Complete prohibition on manufacturing, market placement, and use of PFAS, with no exceptions. | Applies broadly but not adopted as the primary option due to feasibility concerns; serves as a baseline for comparison. |
| RO2: Ban Use Specific Derogations | General ban but allows PFAS use for 5–12 years (plus an 18-month transitional period) in specific applications where viable alternatives are not yet available. Derogations are sector-specific and subject to review. | - Covers all 14 original sectors. - Examples: 5 years for cosmetics; up to 12 years for semiconductors and medical devices. - New derogations for second-hand articles, spare parts, recovered paper/board, and textiles/plastics above concentration thresholds. |
| RO3: Conditional Use Under Restrictions | Allows PFAS use under stringent emission limits over the full life cycle and was considered for a few sectors | - Focused on high-tech sectors like electronics, energy, and PFAS production. - Includes unlimited derogations for research & development (R&D) and intermediates in derogated processes. |
The restriction options RO1, RO2 and RO3 are considered sufficiently enforceable. RO1 is unlikely to be workable or manageable, whereas RO2 and RO3 are concluded to be feasible and practical in relation to implementation, enforcement and management. Complementary measures have also been considered in combination with RO2 to strengthen risk management.
| Scope pf PFAS | Requirement | Conditions of restriction |
| Substances on their own | Prohibited | initial 18-month grace period is targeted at uses where alternatives are expected to be viable in the short term For uses with a high medium-term substitution potential: 6.5 years after date of entry into force (EIF) For uses with a low medium-term substitution potential : 13.5 years after date of EIF Plastic articles containing recovered material, except food contact materials (FCMs) and food contact packaging, and toys: 23.5 years after date of EIF Time-unlimited derogations for paper and board articles containing recovered material except FCMs and packaging |
| Constituents of another substance In mixtures In articles | 25 ppb for individual PFAS (targeted analysis) 250 ppb for the sum of PFAS (with optional precursor degradation) 50 ppm for total fluorine content (including polymeric PFAS) |
Where validated methods are unavailable, total fluorine analysis is used, with the burden on manufacturers or importers to prove whether the fluorine is or is not from PFAS. All three thresholds apply uniformly to any product, with exceedance permitted only where the product qualifies for a specific derogation set out in the draft restriction.
3. Next Steps
Following the publication of the Background Document, ECHA published a note on 27 August 2025 which provides an update on its assessment of proposal and clarifies the excepted timeline.
The proposal is now under evaluation by ECHA's scientific committees, with opinions expected in 2026. RAC and SEAC plan to conclude their discussions on the 14 sectors covered by the original restriction proposal plus PFAS manufacturing and horizontal issues by the end of 2025. ECHA will not evaluate the eight additional sectors included in the updated proposal. This is to allow ECHA to finalize the RAC opinion and the SEAC draft opinion and to carry out the consultation on the SEAC draft opinion in the first half of 2026.
(Remark: according to the updated post from ECHA dated 15 September 2025, SEAC intends to agree its draft opinion at its meeting, which is provisionally scheduled for the first half of March 2026.)
The final RAC and SEAC opinions, together with the Background Document, are expected to enable the European Commission to make informed decisions on how best to address the various use sectors (14 identified sectors plus eight additional ones), PFAS production, and overarching cross-sectoral issues.
The European Commission has committed, in its 8 July 2025 chemical industry action plan, to present a legislative proposal “as soon as possible” after receiving ECHA’s opinions.
France released a notice to reduce the substances of the “Annex VIII authorized list of substances subject to dossier submission before 1 July 2025” of Decree of 5 August 2020 on rubber material used in Food Contact Materials and Pacifiers.
On 11 August 2020, the French government officially published the Decree of 5 August 2020 aimed at regulating rubber materials and articles intended for contact with food and pacifiers for young children. The Decree of 5 August 2020 replaces and repeals previous Decree of 9 November 1994.
Besides the articles, there are eight Annexes of the Decree of 5 August 2020:
Annex I Permitted Monomers, starting substances and modifying agents
Annex II permitted additives
Annex III Test conditions for the measurement of specific migration and global migration
Annex IV N-nitrosamines
Annex V Declaration of Conformity
Annex VI Free volatile organic matter
Annex VII Purity criteria for dyes and pigments
Annex VIII List of authorized constituents subject to the submission of the dossier necessary for their evaluation before 1 July 2025
There are already sixty substances listed in above Annex VIII.
On 7 August 2025, the government of France issued the notice to reduce the authorized substances from 60 to 10 of above Annex VIII. The substances in Annex VIII, but not included in the notice, may no longer be used unless they meet the requirements of Article 10 of the Decree of 5 August 2020 that substances have been evaluated or recognized by the European Food Safety Authority, or from a competent scientific body in the European Union (EU), Turkey or European Economic Area (EEA).
This notice takes effect immediately. It is imperative for relevant businesses to know about the updated regulatory requirements in their production processes to meet compliance.
Noticias de China
In China, when hazards are identified in consumer products, they will be recalled and published in the SAMR Defective Product Administrative Centre, which is updated daily. The China recalls from 01 August 2025 to 31 August 2025 are summarized below:
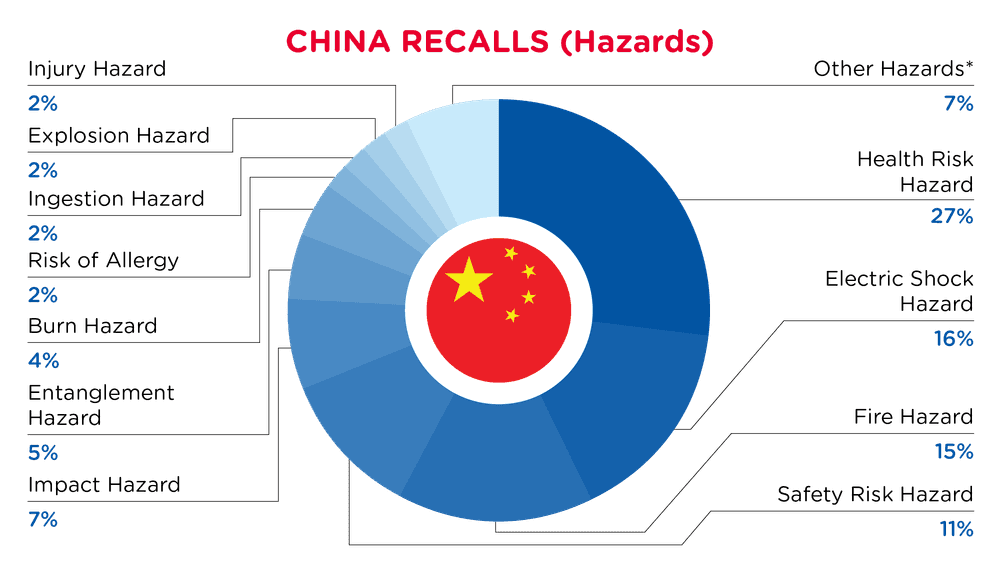
| Hazards | Frequency |
| Health Risk Hazard | 22 |
| Electric Shock Hazard | 13 |
| Fire Hazard | 12 |
| Safety Risk Hazard | 9 |
| Impact Hazard | 6 |
| Entanglement Hazard | 4 |
| Burn Hazard | 3 |
| Risk of Allergy | 2 |
| Ingestion Hazard | 2 |
| Explosion Hazard | 2 |
| Injury Hazard | 2 |
| Other Hazards* | 6 |
*Other Hazards include Crash Hazard, Damage to Sight, Tip-Over Hazard, Laceration Hazard, Puncture Hazard and Suffocation Hazard with a frequency of less than 2.
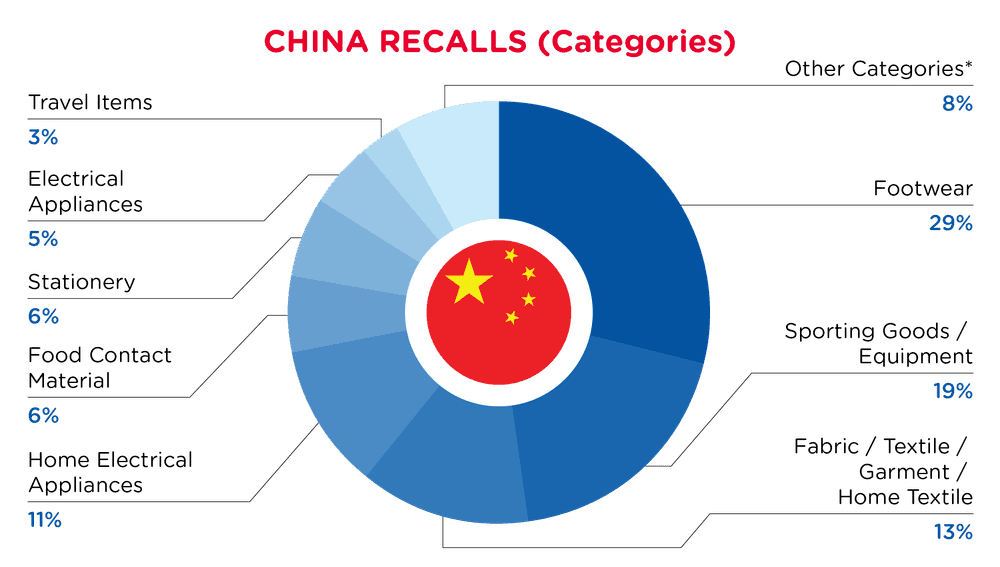
| Product Categories | Frequency |
| Footwear | 18 |
| Sporting Goods / Equipment | 12 |
| Fabric / Textile / Garment / Home Textile | 8 |
| Home Electrical Appliances | 7 |
| Food Contact Material | 4 |
| Stationery | 4 |
| Electrical Appliances | 3 |
| Travel Items | 2 |
| Other Categories* | 5 |
*Other Categories include Toys and Childcare Products, Jewelry, Chemicals, Furniture and Protective Equipment with a frequency of less than 2.
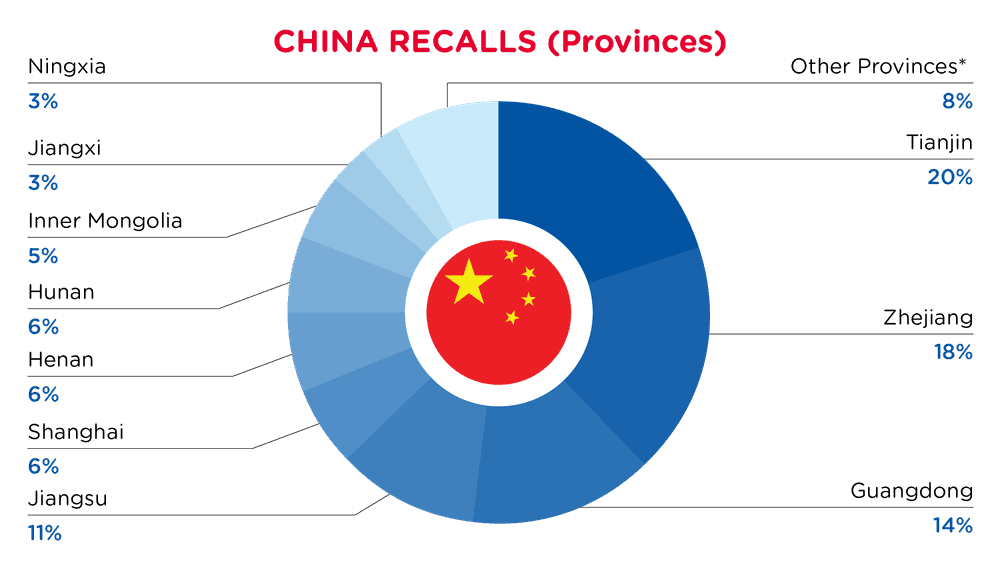
| Provinces | Frequency |
| Tianjin | 12 |
| Zhejiang | 11 |
| Guangdong | 9 |
| Jiangsu | 7 |
| Shanghai | 4 |
| Henan | 4 |
| Hunan | 4 |
| Inner Mongolia | 3 |
| Jiangxi | 2 |
| Ningxia | 2 |
| Other Provinces* | 5 |
*Other Provinces include Chongqing, Gansu, Beijing, Sichuan and Shanxi with a frequency of less than 2.
For a complete list, click here
On 1 August 2025, China formally released GB 26572-2025, "Restrictions on the Use of Hazardous Substances in Electrical and Electronic Equipment". It is China’s first mandatory RoHS standard and will take effect on 1 August 2027, fully replacing the previous GB/T 26572-2011 standard. The new standard aims to reduce pollution to the environment and harm to humans, and to promote the industry’s high-quality development toward “greener” and healthier directions. The standard applies to electrical and electronic products manufactured, sold, and imported within China.
GB 26572-2025 "Restrictions on the Use of Hazardous Substances in Electrical and Electronic Equipment" is China's first mandatory national standard in the Restriction of Hazardous Substances (RoHS) field. It fully replaces the previous recommended standard GB/T 26572-2011, "Limits of Restricted Substances in Electrical and Electronic Products”, marking that China's control of hazardous substances in electrical and electronic products has entered the mandatory phase. The standard is intended to move the industry’s high-quality development in a “greener” and healthier direction.
The standard was approved and issued by the Standardization Administration of China on 1 August 2025 and will come into force on 1 August 2027. A 13-month grace period is provided after implementation (until 1 September 2028) to allow companies to deplete existing inventory.
1. Scope
This document specifies the limit values for hazardous substances in electrical and electronic products and the labeling requirements.
This document applies to electrical and electronic products manufactured, sold, and imported within the territory of the People's Republic of China.
2. Core changes to the standard
2.1 Control substance expansion
Four additional phthalates have been added (DBP, DIBP, BBP, DEHP). The total number of controlled substances increases from 6 to 10, with limits fully aligned with EU RoHS. The full list is:
| Item | Substance Name | CAS number | Limit % |
| 1 | Lead (Pb) | 7439-92-1 | ≤0.1 |
| 2 | Mercury (Hg) | 7439-97-6 | ≤0.1 |
| 3 | Cadmium (Cd) | 7440-43-9 | ≤0.01 |
| 4 | Hexavalent chromium (Cr(VI)) | 18540-29-9 | ≤0.1 |
| 5 | Polybrominated biphenyls (PBBs) | --- | ≤0.1 |
| 6 | Polybrominated diphenyl ethers (PBDEs) | --- | ≤0.1 |
| 7 | Dibutyl phthalate (DBP) | 84-74-2 | ≤0.1 |
| 8 | Diisobutyl phthalate (DIBP) | 84-69-5 | ≤0.1 |
| 9 | Benzyl butyl phthalate (BBP) | 85-68-7 | ≤0.1 |
| 10 | Bis(2‑ethylhexyl) phthalate (DEHP) | 117-81-7 | ≤0.1 |
2.2 Refined classification management
Category I products (e.g., refrigerators, mobile phones and other 12 listed product types): required to comply with both concentration limits and marking requirements.
Category II products (other electrical and electronic products): conformity with concentration limits is encouraged; only marking/labeling obligations are mandatory.
2.3 Testing and marking upgrades
The GB/T 39560 series (equivalent to IEC 62321 on Chemical Analysis of Electronics) is adopted uniformly as the testing method, improving result consistency.
Digital marking options such as Quick-response (QR) codes and electronic displays are added to reduce compliance costs for enterprises.
2.4 Updates to terminology
New terms have been added, e.g., “environmental protection service life” and “catalog of products meeting hazardous‑substance restriction requirements,” clarifying the product’s environmental responsibility lifecycle.
3. Enforcement
| Requirement | Date | |
| Restrictions on the Use of Hazardous Substances in Electrical and Electronic Equipment | Products manufactured after the standard’s effective date must meet the standard’s testing requirements. | 1 August 2027 |
| Sell through of existing stock | For products manufactured or imported before the implementation of the standard, the requirements of this document shall take effect starting from the 13th month after the standard’s implementation. | Allowed until 1 September 2028 |
Noticias de Australia/Nueva Zelanda
In Australia, when hazards are identified in consumer products, they will be recalled and published in the Recalls and Safety Alerts Database on the Australian Competition & Consumer Commission website, which is updated daily. The Australia recalls from 01 August 2025 to 31 August 2025 are summarized below:
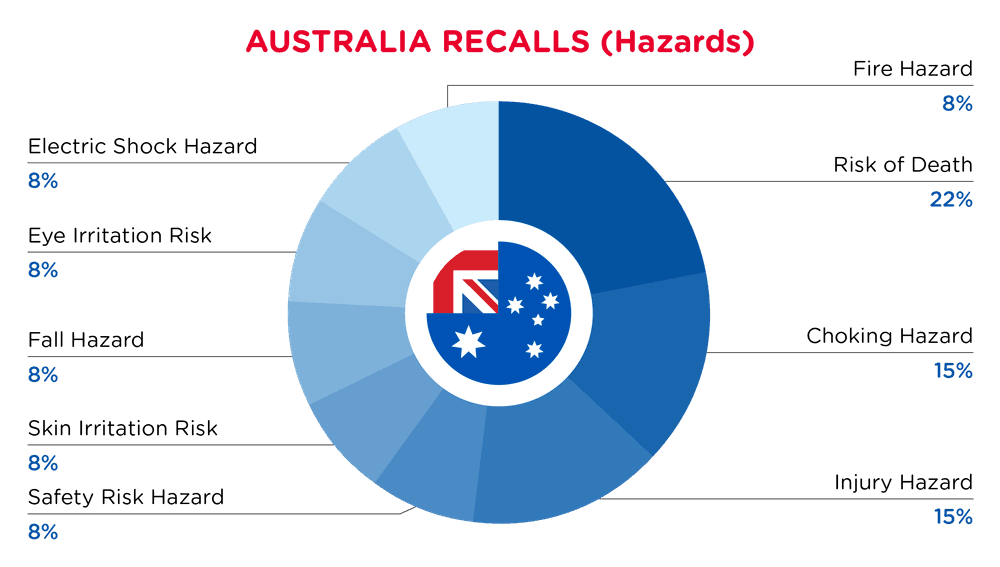
| Hazards | Frequency |
| Risk of Death | 3 |
| Choking Hazard | 2 |
| Injury Hazard | 2 |
| Safety Risk Hazard | 1 |
| Skin Irritation Risk | 1 |
| Fall Hazard | 1 |
| Eye Irritation Risk | 1 |
| Electric Shock Hazard | 1 |
| Fire Hazard | 1 |
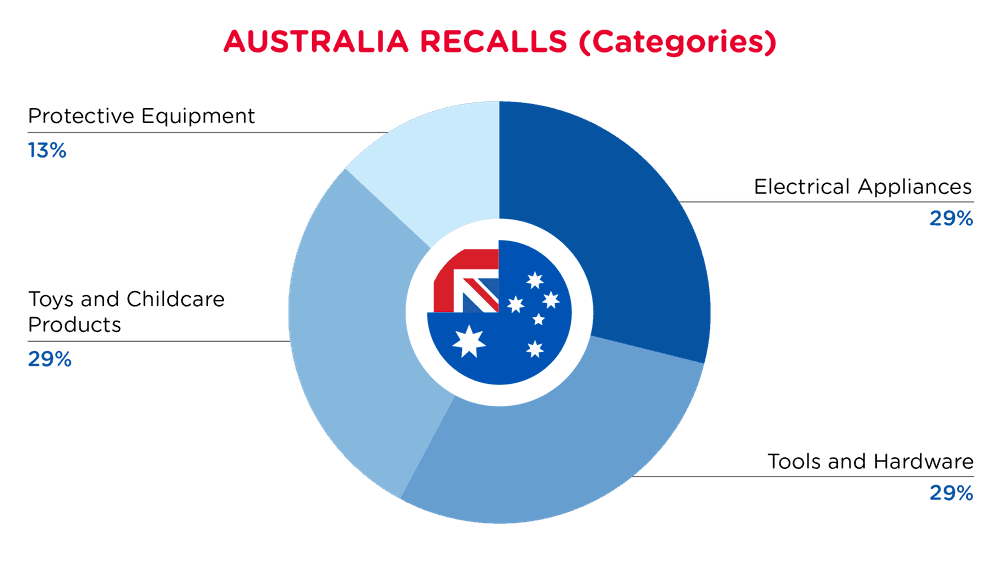
| Product Categories | Frequency |
| Electrical Appliances | 2 |
| Tools and Hardware | 2 |
| Toys and Childcare Products | 2 |
| Protective Equipment | 1 |
For a complete list, click here
Suscríbete a nuestras actualizaciones regulatorias
Anule su suscripción en cualquier momento. Lea nuestra política de privacidad.